
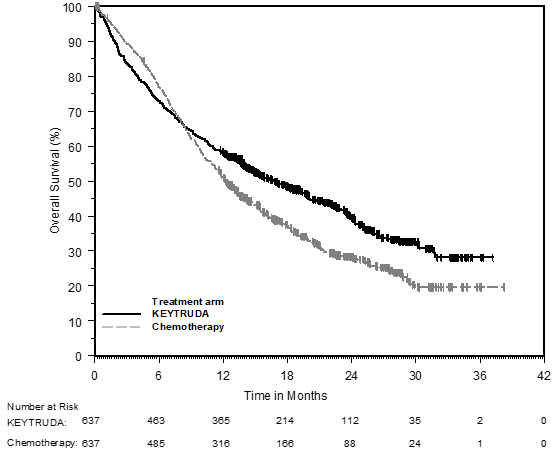
Median (range) time from randomization to data cutoff (June 1, 2020) was 59.9 (55.1-68.4) months. Three hundred five patients were randomly assigned: 154 to pembrolizumab and 151 to chemotherapy.

The primary end point was progression-free survival OS was a secondary end point. Patients in the chemotherapy group with progressive disease could cross over to pembrolizumab. Previous analyses showed pembrolizumab significantly improved progression-free survival and overall survival (OS).Įligible patients were randomly assigned (1:1) to pembrolizumab (200 mg once every 3 weeks for up to 35 cycles) or platinum-based chemotherapy.
#Keynote 024 trial trial#
KEYNOTE-024 ( identifier: NCT02142738) is an open-label, randomized controlled trial of pembrolizumab compared with platinum-based chemotherapy in patients with previously untreated NSCLC with a programmed death ligand-1 (PD-L1) tumor proportion score of at least 50% and no sensitizing EGFR or ALK alterations. We report the first 5-year follow-up of any first-line phase III immunotherapy trial for non-small-cell lung cancer (NSCLC). 17 Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.15 UC Davis Comprehensive Cancer Center, Sacramento, CA.14 Carbone Cancer Center, University of Wisconsin, Madison, WI.13 Okayama University Hospital, Okayama, Japan.12 MedStar Franklin Square Hospital, Baltimore, MD.11 The Royal Marsden Hospital, Sutton, Surrey, UK.10 St James's Hospital and Cancer Trials Ireland (formerly ICORG-All Ireland Cooperative Oncology Research Group), Dublin, Ireland.9 Wollongong Private Hospital and University of Wollongong, Wollongong, NSW, Australia.8 Soroka Cancer Center, Ben Gurion University, Beer Sheva, Israel.7 Meir Medical Center, Kfar-Saba, Israel.6 Országos Korányi Pulmonológiai Intézet, Budapest, Hungary.
5 Jász-Nagykun-Szolnok County Hospital, Szolnok, Hungary.4 Westmead Hospital and the University of Sydney, Sydney, NSW, Australia.3 Cancer Centre of Southeastern Ontario at Kingston General Hospital, Kingston, ON, Canada.2 Complejo Hospitalario Universitario Insular Materno-Infantil de Gran Canaria, Universidad de Las Palmas de Gran Canaria, Las Palmas, Spain.1 Lung Clinic Grosshansdorf, Airway Research Center North (ARCN), member of the German Center for Lung Research (DZL), Grosshansdorf, Germany.


 0 kommentar(er)
0 kommentar(er)
